Source Study
About Source Study
In the context of health research, datasets are closely related to the research or study and serve as a valuable source of information. Hence in HDA, every health dataset is related to a study. Currently, the available study type primarily revolves around clinical trials, sourced from Australian New Zealand Clinical Trials Registry (ANZCTR). This section provides insights into the context of the study associated with the dataset. The metadata fields pertaining to the study are grouped into different tabs, ensuring easy navigation and access to specific information of your interest. Certain fields within the tabs are also faceted, allowing you to click on these fields and explore related datasets that share the same attribute.
Summary
The Source Study contains three tabs. The first tab, Summary, provides key details about the trial, including its identification, funding, eligibility criteria, and a brief overview.
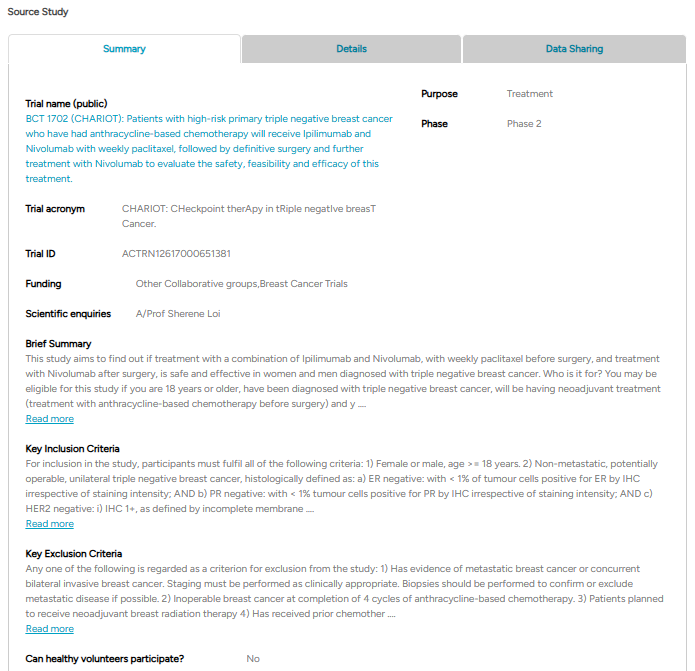
Fig 4.1: An example of a source study ‘Summary’ section
Details
The second tab, Details, covers information on the study population, interventions, comparisons, and outcomes. This structure aligns with the PICO framework, facilitating HDA users to quickly identify the most relevant evidence.
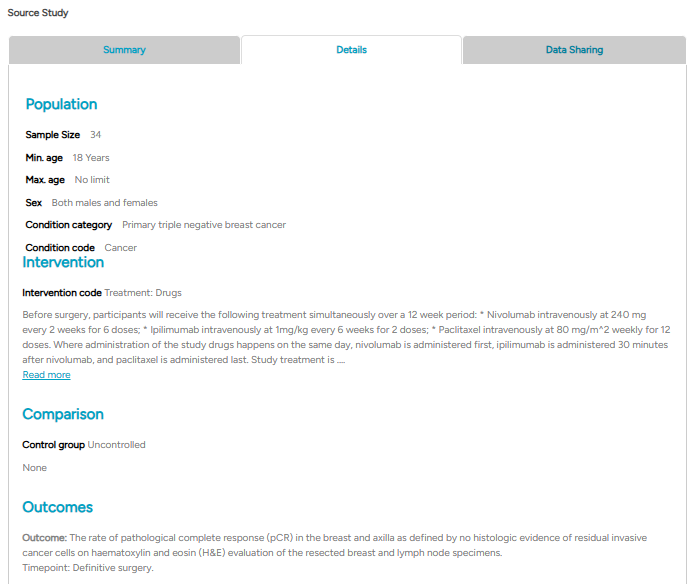
Fig4.2: An example of a source study ‘Details’ section
Data Sharing
The third tab, Data Sharing, provides details on the study protocol, data availability, sharing scope, and conditions for access and analysis.
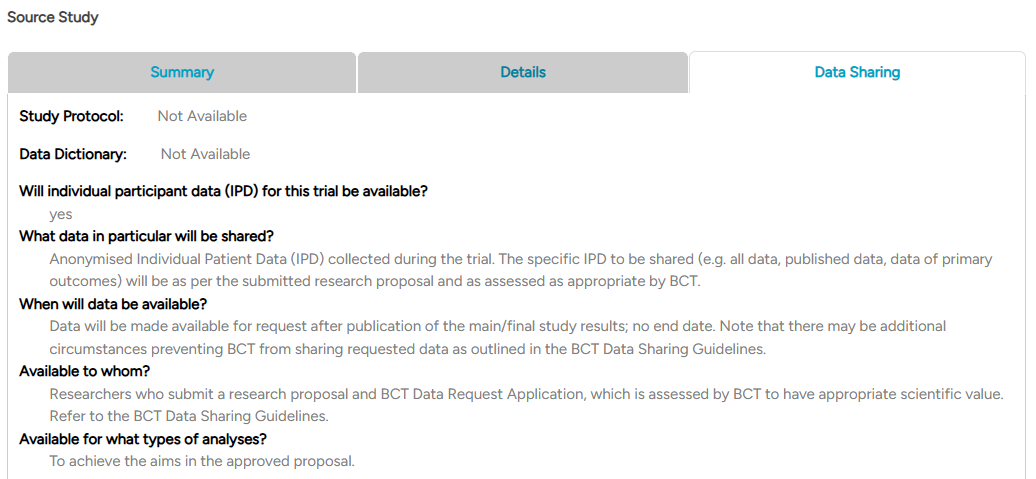
Fig4.3: An example of a source study ‘Data Sharing’ section
If no data-sharing information is available for a study, the Data Sharing tab will display the message: "The data-sharing statement for this study is currently unavailable."
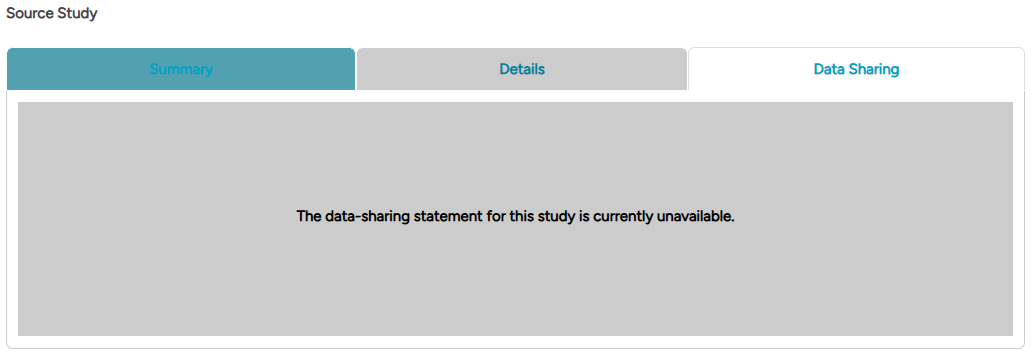
Fig4.4: An example of a source study's Data Sharing section with the data-sharing statement currently unavailable.
